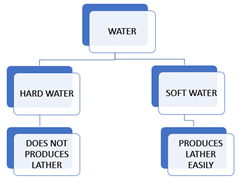
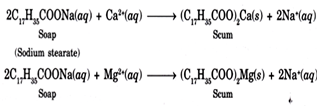
The term “hardness of water” was originally coined to describe the difficulty of making a soap lather with water that contains certain ions.

The hardness of water is a measure of the concentration of dissolved minerals, primarily calcium and magnesium ions.
These minerals are commonly present in water as bicarbonates, sulfates, and chlorides.
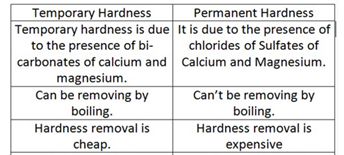
The salts responsible for the hardness of water are primarily the salts of calcium and magnesium. The two main types of hardness, temporary and permanent, are associated with different salts:
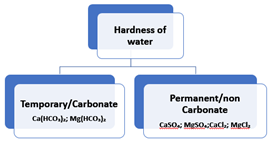
- Temporary Hardness:
- Calcium bicarbonate (Ca(HCO₃)₂)
- Magnesium bicarbonate (Mg(HCO₃)₂)
These bicarbonate ions are responsible for temporary hardness because they can be easily removed by boiling the water, causing them to decompose and form insoluble carbonate precipitates.
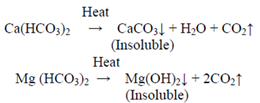
2. Permanent Hardness:
- Calcium sulfate (CaSO₄)
- Magnesium sulfate (MgSO₄)
- Calcium chloride (CaCl₂)
- Magnesium chloride (MgCl₂)
These salts contribute to permanent hardness as they do not decompose upon boiling. Permanent hardness often requires the use of water softeners or other chemical treatments to reduce the concentration of calcium and magnesium ions.
In both cases, the overall hardness is often expressed in terms of calcium carbonate (CaCO₃) equivalents. This is done to simplify communication and calculations related to water treatment. The chemical reactions involved in the removal of temporary hardness by boiling typically result in the precipitation of calcium carbonate (CaCO₃) and magnesium hydroxide (Mg(OH)₂).
The hardness of water can have practical implications, especially in industrial processes, household activities, and the performance of water-related appliances. Water with high hardness may lead to the buildup of scale in pipes and appliances, reducing their efficiency and lifespan. Additionally, it can affect the lathering of soap and detergent in cleaning processes.

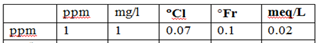
Water hardness is commonly measured in milligrams per liter (mg/L) or parts per million (ppm) of calcium carbonate. The specific hardness levels are often classified as follows:
- Soft water: 0 – 60 mg/L (or ppm) CaCO₃
- Moderately hard water: 61 – 120 mg/L CaCO₃
- Hard water: 121 – 180 mg/L CaCO₃
- Very hard water: > 180 mg/L CaCO₃
Water softeners are often used to reduce the hardness of water by exchanging calcium and magnesium ions with sodium ions, which do not contribute to water hardness.