The degree of hardness of water refers to the concentration of dissolved minerals, primarily calcium and magnesium ions, in water. These minerals are the main contributors to water hardness. Hard water can cause various issues, such as scaling in pipes, reduced soap efficiency, and potential damage to appliances like water heaters.
Why Hardness is usually expressed in terms of CaCO3 equivalent? Expressing the hardness of water in terms of calcium carbonate (CaCO₃) equivalents is a convention that simplifies the communication of water quality information. Here’s why this approach is commonly used:
Common Reference Point: Calcium carbonate is a common mineral, and its chemical formula is CaCO₃. It helps to relate the hardness of water to a substance that is widely recognized.Equivalent Mass: Calcium carbonate has a molar mass of 100.09 g/mol. When expressing hardness in CaCO₃ equivalents, it is as if the calcium and magnesium ions in the water were replaced by an equivalent amount of calcium carbonate. This simplification allows for a straightforward conversion from the concentration of calcium and magnesium ions to an equivalent concentration of CaCO₃, making it easier to compare and understand.Consistency in Reporting: Using CaCO₃ equivalents provides a standardized and consistent way to report water hardness across different locations and sources.Practical Impact: The expression in CaCO₃ equivalents helps convey the practical impact of water hardness on common activities.
How can we calculate or express the hardness of water in terms of CaCO₃ equivalents?
Let’s take an example of hardness producing substance (HPS) CaCl2 and a hardness representative salt i.e. calcium carbonate (CaCO₃) reacting with soap i.e. sodium stearate (C17H35COONa).
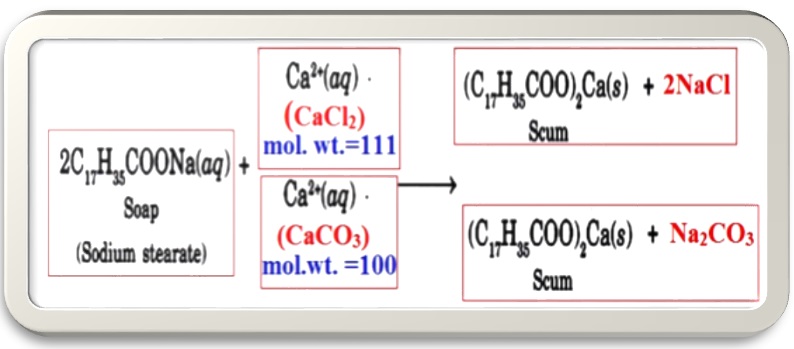
From the above reaction it can be seen that 02 moles of soaps are reacting with 01 mole of CaCl2 and CaCO3 to form scum. This formation of Ca-stearate can be considered as consumption of soap by these salts.
Since, 01 mole of CaCl2 is equal to 111gm and 01 mole of CaCO3 is equal to 100gm it can also be concluded that 111gm CaCl2 and 100gm CaCO3 both are reacting with 02moles of soap.
It means,
When 111gm CaCl2 = 100gm CaCO3
Than ‘x’ gm of CaCl2 will be = 100/111 * xgm of CaCl2
Or
We can say that ( 100/111* x gm of CaCl2) in terms ofCaCO3
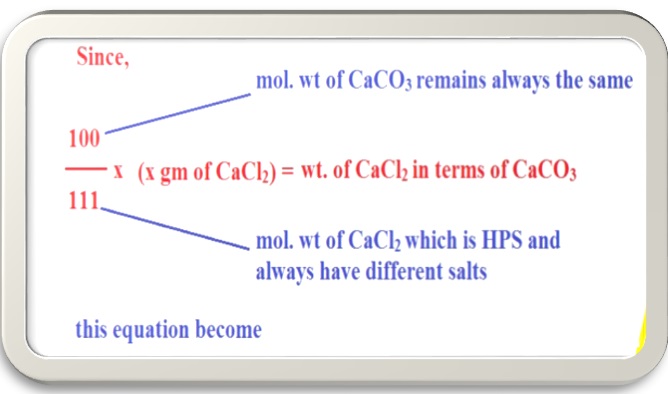
Since, we have many dissolved salts responsible for hardness of water and need to be converted into CaCO3 equivalents because the hardness of water due to all dissolved salts of Ca and Mg are represented in terms of CaCO3 equivalents.

But the above mentioned case is a particular case of CaCl2. Where, both CaCl2 and CaCO3 has the n-factor of 02. (n-factor=02). But there are many salts whose n-factor is different from CaCO3 like Al2(SO4)3 has n-factor of 06.Since, we are converting all salts into CaCO3 equivalents whose n-factor is 02, then we can generalize the above equation as,
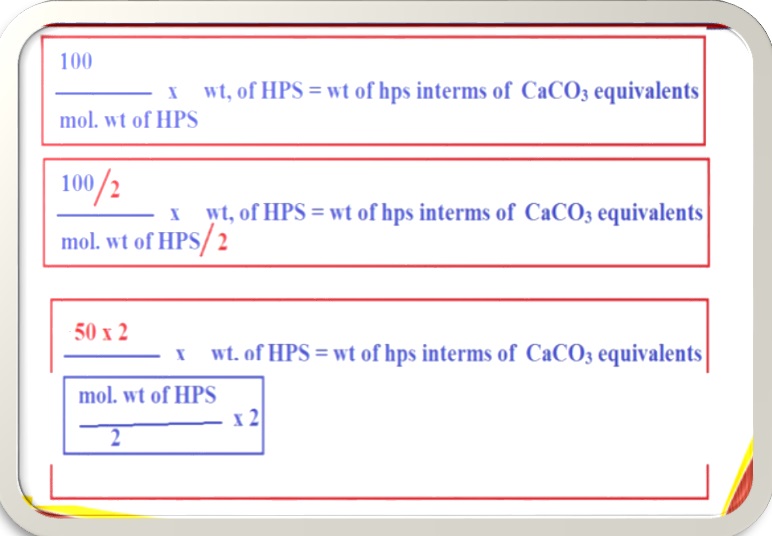
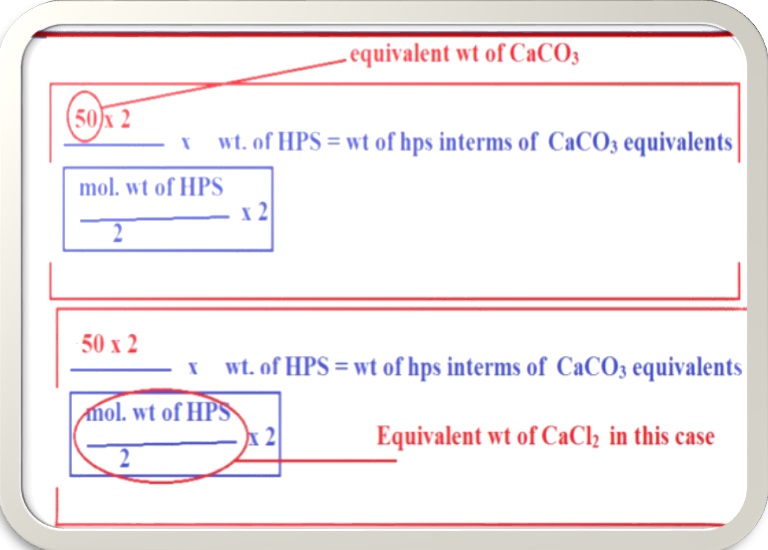

For example. If we want to express the hardness of water if 20mg/l of CaSO4 is present in water sample. Then we have to multiply 20mg/l of CaSO4 with the above generalized equation known as multiplication factor.
