The determination of hardness in water is a crucial parameter for various industrial and domestic applications. Hardness is primarily caused by the presence of divalent cations, mainly calcium (Ca2+) and magnesium (Mg2+). The ethylene diamine tetra acetic acid (EDTA) titration method is widely employed to quantify hardness due to its high precision and reliability.


Since pure EDTA is insoluble in water, Di sodium salts of EDTA is used which gives off 2Na+ and get ionize. This is denoted as H2Y-2.
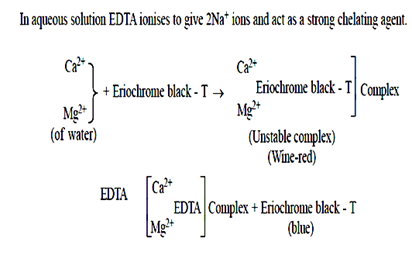
The indicator used is a complex organic compound (sodium – 1 – (1-hydroxy 2-naphthylazo)-6-nitro-2-naphthol-4-sutphonate), commonly known as Eriochrome black T(EBT). It has two ionisable phenolic hydrogen atoms and for simplicity it is represented as Na+H2In–:
Principle of EDTA Titration for Hardness Determination:
EDTA is a versatile chelating agent that forms stable complexes with metal ions. In the context of water hardness determination, the reaction involves the complexation of metal ions (Ca2+ and Mg2+) with EDTA. The balanced chemical equation for the reaction with calcium ions is:
Procedure:
- Sample Collection: A water sample is collected and acidified to prevent interference from other metal ions.
- Complex Formation: A buffer solution with a known pH (typically around 10) is added to the sample. This maintains the pH at a level where the metal-EDTA complex formation is optimal. The buffer also prevents the interference of other metal ions.
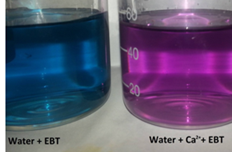
- Titration: A standardized EDTA solution is slowly added to the sample until the endpoint is reached. The endpoint is marked by a color change, usually from wine-red to blue, facilitated by the addition of a metal indicator such as Eriochrome Black T or Calmagite.

4. Calculation: The amount of EDTA required to titrate the metal ions is used to calculate the hardness. The concentration of metal ions can be determined using the stoichiometry of the reaction and the volume of the EDTA solution used.
Chemical Reactions Involved:
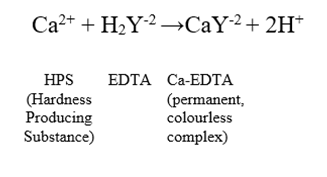
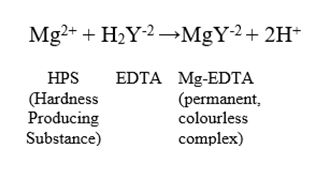
Here, H2Y-2 represents the EDTA molecule. Similar reactions occur with magnesium ions. The stoichiometry of these reactions is 1:1, meaning one mole of metal ion reacts with one mole of EDTA.
These reactions demonstrate the formation of metal-EDTA complexes. The color change during titration is due to the liberation of the metal ions from their complexes with EDTA, resulting in the indicator’s color change.
Advantages of EDTA Titration:
- Selectivity: EDTA has a high selectivity for calcium and magnesium ions, minimizing interference from other ions.
- Sensitivity: The method can detect low concentrations of metal ions with high precision.
- Versatility: The EDTA titration method can be adapted for both calcium and magnesium determination, providing total hardness.
In conclusion, the determination of water hardness by the EDTA titration method is a reliable and widely accepted technique. The complexation reactions between metal ions and EDTA, coupled with the use of suitable indicators, enable accurate quantification of hardness in water samples, crucial for various industrial and domestic purposes.