The water present on Earth is divided into 3 categories: salt water, polar ice caps and fresh water. Out of the total water present on earth, 97% of water is salt water or sea water. 2% water is in the form of polar ice caps, so 97% + 2% make it 99%. So the rest 1% which is considered as fresh water which is the only source of water available for our use. It means still after having a huge amount of water on our earth surface, we are only able depend on this 1%.
One question which should come in our mind that, water is a most abundantly available but Still water crisis? There are several reason for this water crisis but one of the main reason is the limited availability of fresh water. But we cannot use that saline water for our domestic and industrial purposes moreover the cost to convert that saline water present in the form of sea water is very costly.
For a person with no strong science background, Water is a transparent, tasteless, and odorless liquid at room temperature. It is a crucial substance for all known forms of life on Earth. The chemical formula for water is H2O.
Chemically, water is composed of two hydrogen (H) atoms with one electron each, covalently bonded to one oxygen (O) atom with six electrons, forming a molecule with the chemical formula H2O.
 The four electron pairs result in a tetrahedral geometry-the angle between electron pairs (and therefore the H-O-H bond angle) is 109.5°. The lp-lp>bp-bp effectively pushing the two hydrogen atoms closer together. The result is a distorted tetrahedral arrangement in which the H—O—H angle is 104.5°.
The four electron pairs result in a tetrahedral geometry-the angle between electron pairs (and therefore the H-O-H bond angle) is 109.5°. The lp-lp>bp-bp effectively pushing the two hydrogen atoms closer together. The result is a distorted tetrahedral arrangement in which the H—O—H angle is 104.5°.
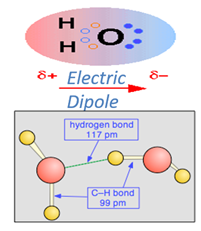
Its eight electrons are not distributed uniformly; there is slightly more negative charge (purple) at the oxygen end of the molecule, and a compensating positive charge (green) at the hydrogen end. The resulting polarity is largely responsible for water’s unique properties.

Partially-positive hydrogen atom on one water molecule is electrostatically attracted to the partially-negative oxygen on a neighboring molecule. This process is called (somewhat misleadingly) Hydrogen Bonding.
- The oxygen atom attracts electrons more strongly than the hydrogen atoms, creating a polar molecule. This polarity gives water its unique properties to be called as universal solvents, these properties include such as the ability to dissolve a wide variety of solutes, high surface tension, and a relatively high heat capacity. The arrangement of atoms in a water molecule is responsible for its essential role in supporting life and various natural processes.