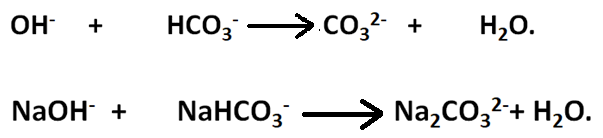Alkalinity is a crucial water quality parameter that measures the capacity of water to neutralize acids, thereby maintaining its pH within a certain range.
Since the bicarbonate, carbonate, and hydroxide ions are able to neutralize acid they causes alkalinity of water. It is a key factor in determining the overall health and stability of aquatic ecosystems and is of significant importance in various industrial and environmental applications. Alkalinity is often associated with the presence of bicarbonate, carbonate, and hydroxide ions in water.
With respect to the simultaneous presence of the constituents causing alkalinity in natural water the following possibilities may arise
1. Hydroxides
2. Carbonates
3. Bi carbonates
4. Hydroxides and Carbonates
5. Carbonates and Bicarbonates

The possibilities of hydroxides and bicarbonates existing together is rules out owing to the fact that they combine with each other forming the respective carbonates